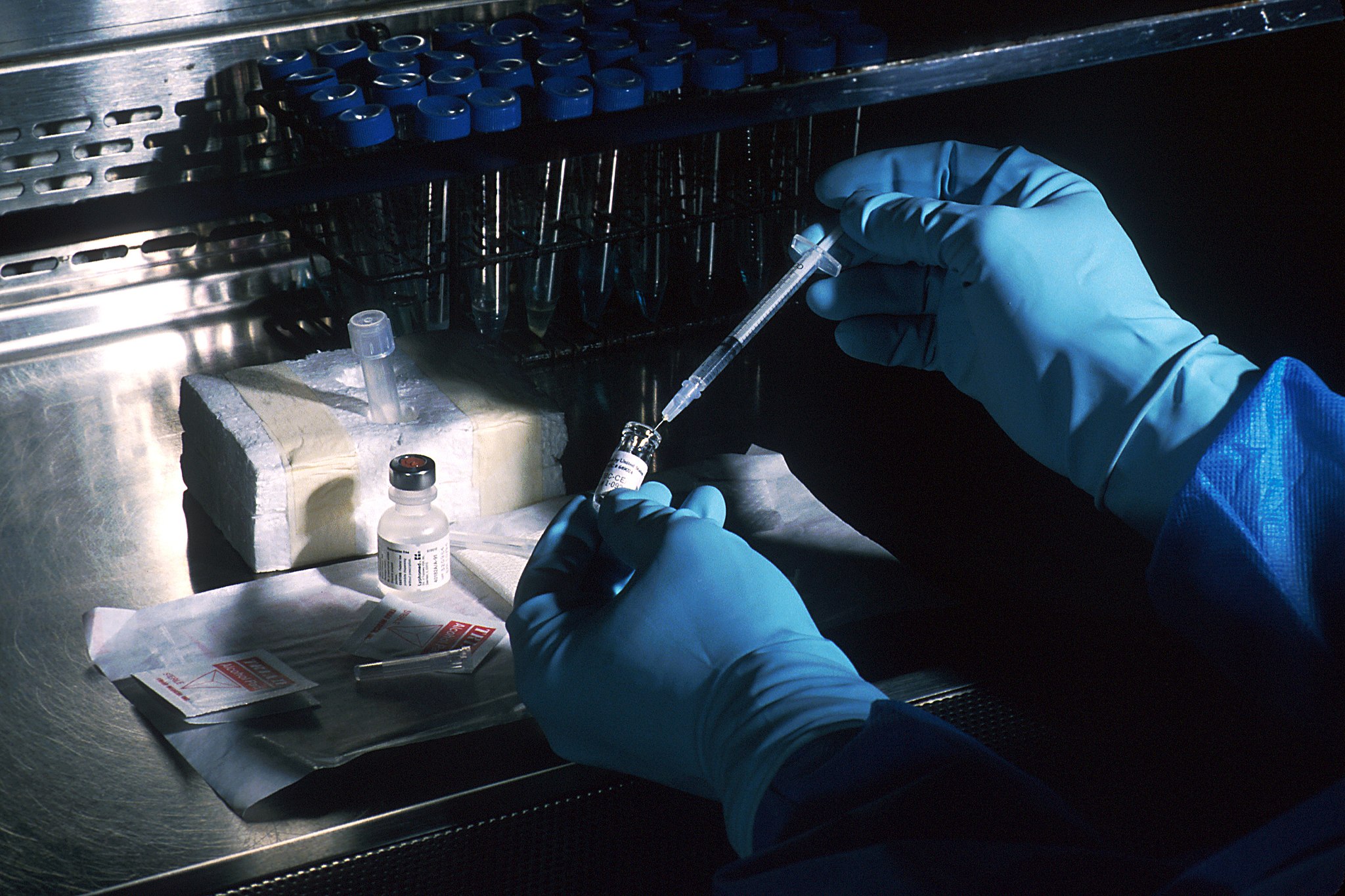
The typical process for developing a vaccine — of rigorous rounds of trials and long evaluation periods — is not going to work for COVID-19. Saving lives and livelihoods depends on significantly speeding up the process and evaluating the risk of that.
Dr. Penny Heaton, the CEO of the Bill and Melinda Gates Medical Research Institute, has written about this compression of six years of work into six months. Dr. Heaton joined the Texas Standard to discuss COVID-19 vaccine development.
What factors have enabled the unprecedented speed of vaccine development?
“We already understood how it causes disease and what we need to target with respect to a vaccine to try to prevent coronavirus disease. The second was just the new vaccine technologies that have emerged over the last several years allow production of thousands of doses for clinical trials very quickly. And then the third was really figuring out activities in parallel that are not done sequentially.”
Are there additional risks to moving forward with this vaccine?
“In the normal course of vaccine development, we are looking at things like what is the unmet medical need, how fast can we move forward and what are the benefits of moving forward versus the potential risk of this particular vaccine. The vaccine developers are thinking about that same ratio of benefit risk here.”
Will it continue to evolve, even once the trial period is complete?
“The vaccine developers will continue on with what we call life-cycle management. So even when vaccines are approved, the safety will continue to be monitored post-approval. So these questions that you can only answer with time, and only answer as thousands and millions of people get the vaccine, they will still be answered.”
How long before a vaccine will be readily available to the public?
“I am not directly involved with development of any of these vaccines, so any estimates that I give you would only be speculation. But I am optimistic from what I’m hearing from colleagues and scientists, etc. that, in the next six-to-twelve months, that the first vaccine doses could be available.”
Web story by Sarah Gabrielli
If you found the reporting above valuable, please consider making a donation to support it here. Your gift helps pay for everything you find on texasstandard.org and KUT.org. Thanks for donating today.